Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
A 6-Year-Old Boy with Generalized Weakness and Inability to Walk
*Corresponding author: Corinne McCabe, Nationwide Children’s Hospital and The Ohio State University College of Medicine, 700 Children’s Drive, Columbus, OH 43205, USA.
Received:July 17, 2022; Published: July 25, 2022
DOI: 10.34297/AJBSR.2022.16.002281
Abstract
A 6-year-old, non-verbal boy with global developmental delay and autism presented with acute onset of weakness, inability to walk, and decreased energy. His exam was consistent with an upper motor neuron lesion that localized to the cervical spinal cord. MR spine showed extensive edema involving the medulla and upper cervical cord to the C5 level and intraparenchymal spinal cord hemorrhage at the C1-C2 level with enhancement. MR brain showed mild bilateral optic/perioptic enhancement. Therefore, the impression was concerning for autoimmune or para-infectious myelitis and he was initially treated with high-dose steroids. CSF studies, infectious work-up, and autoimmune antibodies were negative. Unexpected and significant improvement of motor function and energy within 48 hours of admission, as well as concern that spinal cord hemorrhage was unusual for inflammatory disease, led to re-evaluation for underlying etiology. A CT head and neck was obtained and showed the ultimate diagnosis of atlantoaxial subluxation with os odontoideum which was treated with surgical fixation. It is important to recognize when unexpected progression of a clinical presentation or unusual features of radiographic findings warrant re-evaluation. This is especially pertinent when caring for our most vulnerable children with complex medical histories and developmental delay including limited verbal communication leading to difficulty interpreting history, symptoms, and exam findings.
Section 1
A case report of a 6-year-old, non-verbal boy with Potocki-Lupski Syndrome manifesting as global developmental delay, autism spectrum disorder, and self-injurious behaviors presented to the emergency department with lower extremity weakness. Four weeks prior to presentation he was treated for right acute otitis media and since then has seemed to hold his head down and to the right. One week prior to presentation, while sitting on the ground, he fell and struck his head. A few days later he developed difficulty with ambulation with a wide-based and unsteady gait. On the day of presentation he was unable to bear weight or sit unassisted, had decreased energy, and had a low-grade fever (100.4F). Initial examination demonstrated a preference to laying still with few spontaneous movements and sometimes looking towards the examiner. He did respond to light touch and simple verbal commands. Upper extremity exam showed anti-gravity left arm movement without a reliable exam against resistance with weak left-hand grip and right arm movement only within the plane of the bed with no appreciable right-hand grip. When resting his legs were held in plantar flexion with increased tone. He had +3 reflexes throughout, except for left patella that was +4 with sustained clonus.
Questions for Consideration:
a.What is the localization of the patient’s symptoms?
b.What is the differential diagnosis?
Section 2
Weakness, increased tone, and hyperreflexia localizes to an upper motor neuron lesion and the bilateral upper and lower extremity findings localizes to the cervical spinal cord. The differential diagnosis for cervical spinal cord lesions includes trauma, infection, ischemia, mass, and demyelinating processes. The patient did have a fall from a seated height prior to presentation and family also endorsed head-banging behavior. There were no signs or symptoms of infection. A demyelinating process was high on the differential because of rapid onset of weakness and possible altered mental status (i.e. decreased energy). The most common demyelinating processes in children are acute demyelinating encephalomyelitis (ADEM), acute transverse myelitis (ATM), neuromyelitis optica spectrum disorder (NMOSD), and myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease. Multiple sclerosis can also be considered but due to patient’s age this was thought to be less likely.
Experimental Procedures
Question for Consideration:
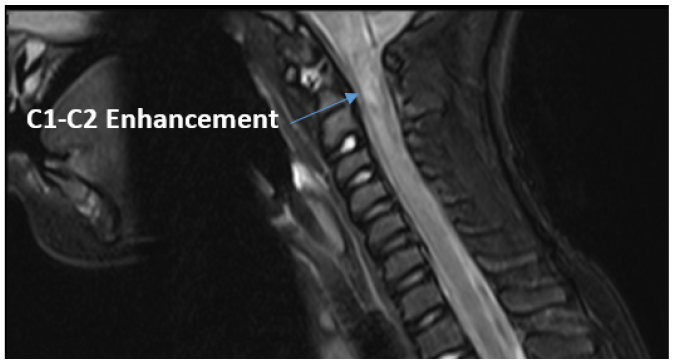
Magnetic Resonance Imaging (MRI) of the head and complete spinal cord showed extensive edema involving the medulla and upper cervical cord to the C5 level, hemorrhage at the C1-C2 level with enhancement, scattered low level enhancement and edema throughout the rest of the cord with conus tip and cauda equina nerve roots mildly thickened, and mild bilateral optic/perioptic enhancement with final impression concerning for autoimmune or para-infectious myelitis (Figure 1). Edema throughout the cervical spinal cord with most prominent enhancement at the C1-C2 levels. These multi-focal findings placed a demyelinating process higher on the differential.
The patient’s CSF studies demonstrated 1 white blood cell/ mm3, a negative PCR panel that included the most common bacterial and viral causes of meningitis and encephalitis, no oligoclonal bands, and IgG Index slightly elevated at 0.68 (reference range 0.28 - 0.66). Serum studies were remarkable for mildly elevated LFTs and elevated creatinine kinase. Further viral and bacterial studies, including enterovirus D68, and serum MOG and AQP4 antibodies were negative.
Questions for Consideration:
a. What treatment would you start with these findings?
b. Do any of the findings make you consider another etiology for
the patient’s symptoms and how would you further investigate
them?
Section 3
The patient was started on high-dose intravenous steroid treatment for acute transverse myelitis.
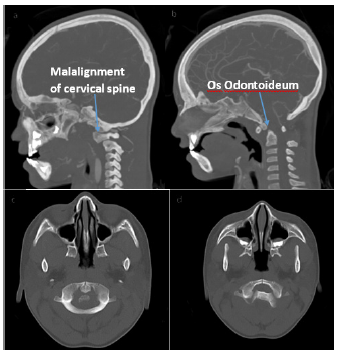
Before initiation of steroids the patient’s neurologic exam started to improve and he and was able to sit up in bed independently. Within 48 hours he was able to ambulate and was near baseline energy level. Due to his unusual clinical course and concern that imaging was inconsistent with transverse myelitis, specifically the hemorrhagic component, further investigation was conducted. A CT head and neck angiogram was performed to look for a vascular injury or anomaly that may have led to the hemorrhage. Results demonstrated suspected unstable cranio-cervical junction with os odontoideum, malalignment, and cervical stenosis (Figure 2). When compared to the recent MRI, the cord signal abnormality may have represented traumatic injury as opposed to inflammation.
Neurosurgery was consulted and believed the findings were from recurrent trauma to the cervico-medullary segment due atlantoaxial subluxation. The patient was placed in a hard c-collar for stabilization and taken to the operating room for C1-C2 spinal fusion. He was discharged home in stable condition.
Discussion
Atlantoaxial subluxation refers to a loss of stability between the atlas and axis (C1-C2) resulting in loss of normal articulation [1]. This disorder is most often seen in adolescents and causes include trauma, inflammation, congenital abnormalities, or idiopathic, but most often it is multifactorial [2]. This patient has Potocki- Lupinski syndrome, which can be associated with connective tissue abnormalities and joint hypermobility, possibly causing a predisposition for atlantoaxial instability [2]. Additionally, his history included repeated head trauma from self-injury behaviors, and a fall from sitting height a few days before presentation. All these factors likely contributed to joint instability that led to subluxation requiring spinal fixation.
The initial working diagnosis for this patient’s clinical and radiographic presentation was acute transverse myelitis (ATM). ATM can be secondary to a primary autoimmune disease (i.e. NMOSD or MOG-associated demyelination), a paraneoplastic disorder, or can be a post-infectious immune mediated disorder (i.e. ADEM). [3]. ATM accounts for approximately 20% of all pediatric patients experiencing their first acquired demyelinating syndrome [3,4]. It affects 1.2-2 children/million/year with a bimodal distribution of those under 5 and those greater than 10 [5]. The most common presenting symptom for ATM is back pain, followed by motor and sensory deficits or bladder/bowel dysfunction. Usually, the initial symptoms evolve over 2-4 days and peak at 5-6 days and, by definition, it reaches maximum deficit within 21 days [3,4]. Approximately 66% of patients have had a prodromal illness within 30 days of presentation [3]. Although ATM seemed to fit the clinical and radiographic presentation, it is important to consider alternative etiologies such as intrinsic and extrinsic disorders of the spinal cord including trauma, vertebral body compression, intervertebral disc herniation, epidural hematoma, arteriovenous malformations, tumors, direct infections, and rheumatologic conditions [3].
Evaluation of a patient with exam findings localizing to the cervical spinal cord includes MRI without and with contrast. Additional work-up includes CBC and chemistry, CSF analysis (including immunoglobulin type G(IgG) index and oligoclonal bands), serum viral and bacterial infectious studies, serum APQ- 4 IgG and MOG antibodies, and consideration for rheumatologic work-up (e.g. dsDNA, ANA, etc.) and vitamin deficiencies (e.g. B12, folate) [3,4]. The diagnosis of ATM can be given when compressive spinal cord lesions have been ruled out and the patient must have confirmation of spinal cord inflammation by either gadolinium enhancing lesion on MRI or CSF evidence of either pleocytosis or elevated IgG index [5]. This patient’s ATM diagnosis was made based off MRI results.
Interestingly, the patient had hemorrhagic features within the spinal cord lesion which is an extremely rare finding in pediatric ATM. One case report noted hemorrhagic longitudinally extensive transverse myelitis (LETM) in a previous healthy 28-year-old women who recently received her influenza vaccination [6]. Hemorrhagic LTEM has been associated with VZV and HSV-2 infections in immunocompromised patients and, very rarely, in immunocompetent individuals. Another known association with hemorrhage in immune-mediated diseases is acute hemorrhagic leukoencephalopathy (AHLE), a rare fulminant subtype of ADEM [7].
ADEM was also considered on the differential diagnosis for this patient. ADEM can occur at any age but is more common in the pediatric population and is usually associated with a defined viral illness or vaccine.8 It presents with rapid encephalopathy in combination with multifocal neurologic deficits [8]. At times during hospitalization the patient’s mental status was considered altered (i.e his decreased energy) but he was never encephalopathic; his AMS was, in hindsight, most likely an expression of pain or frustration in a non-verbal individual. With the patient’s unusual MRI results further investigation including head and neck CT was performed and atlantoaxial subluxation was identified. This case demonstrated that an initial diagnosis is not always correct and oftentimes re-evaluation is needed. Here the imaging and clinical course were re-examined, the correct diagnosis was established, and appropriate treatment was given. When caring for individuals who are unable to express symptoms due to developmental delay, age, or other medical conditions, special attention to caregiver’s history, practioners’ physical exams, and continued re-evaluation of clinical course is necessary.
References
- Yang SY, Boniello AJ, Poorman CE, Chang AL, Wang S, Passias PG. A review of the diagnosis and treatment of atlantoaxial dislocations. Global Spine J. 2014 Aug;4(3):197-210. doi: 10.1055/s-0034-1376371. Epub 2014 May 22. PMID: 25083363; PMCID: PMC4111952.
- Potocki L, Neira-Fresneda J, Yuan B. Potocki-Lupski Syndrome. 2017 Aug 24. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2021. PMID: 28837307.
- Absoud M, Greenberg BM, Lim M, Lotze T, Thomas T, Deiva K. Pediatric transverse myelitis. Neurology. 2016 Aug 30;87(9 Suppl 2):S46-52. doi: 10.1212/WNL.0000000000002820. PMID: 27572861.
- Eoin P. Flanagan, Chapter 19 - Autoimmune myelopathies, Handbook of Clinical Neurology, Elsevier, Volume 133, 2016, Pages 327-351
- Tavasoli A, Tabrizi A. Acute Transverse Myelitis in Children, Literature Review. Iran J Child Neurol. 2018 Spring;12(2):7-16. PMID: 29696041; PMCID: PMC5904733.
- Chris Y. Wu, Tanawan Riangwiwat, Beau K. Nakamoto, “Hemorrhagic Longitudinally Extensive Transverse Myelitis”, Case Reports in Neurologic Medicine, vol. 2016, Article ID 1596864, 3 pages, 2016.
- Waak M, Malone S, Sinclair K, Phillips G, Bandodkar S, Wienholt L, Robertson T, Whitehead B, Trnka P, Kothur K, Dale RC. Acute Hemorrhagic Leukoencephalopathy: Pathological Features and Cerebrospinal Fluid Cytokine Profiles. Pediatr Neurol. 2019 Nov;100:92-96. doi: 10.1016/j.pediatrneurol.2019.06.013. Epub 2019 Jun 28. PMID: 31376926.
- Tenembaum S, Chitnis T, Ness J, Hahn JS; International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology. 2007 Apr 17;68(16 Suppl 2):S23-36. doi: 10.1212/01.wnl.0000259404.51352.7f. PMID: 17438235.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.